Research projects
Functional Analysis of Tetrapeptide Repeat
Proteins in Myotonic Dystrophy Type 2
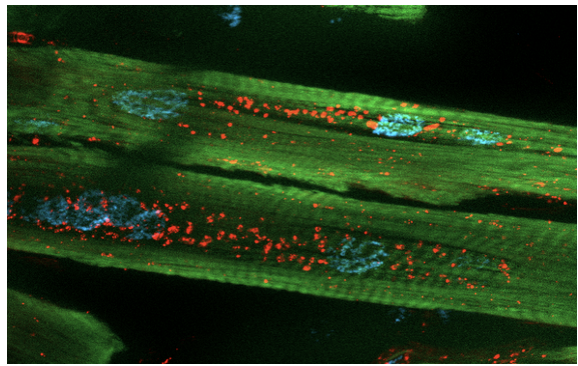
We are investigating the pathogenic roles of poly-QAGR and poly-PACL RAN-translated tetrapeptide repeat proteins (TPRs) in myotonic dystrophy type 2 (DM2). Using Drosophila melanogaster and human cell models, we express codon-optimized peptides to bypass RNA-mediated toxicity and isolate protein-specific effects. Current studies examine their impact on viability, locomotion, and lifespan, and explore distinct mechanisms. QAGR accumulates in the nucleolus, disrupting rRNA processing and autophagy, while PACL associates with stress granules in both flies and patient myoblasts. These complementary pathways may converge in DM2 pathogenesis and represent potential therapeutic targets.
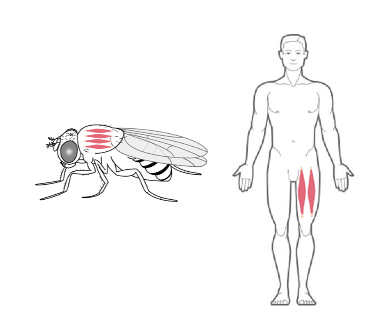
Drosophila as model system for Myotonic Distrophy type 2
Myotonic Dystrophy 2 (DM2) is a genetic multi-systemic disease, primarily affecting skeletal muscle. It is caused by CCTGn expansion in intron1 of the CNBP gene. Our studies are aimed at clarifying important and previously unresolved issues concerning the relative contribution of CNBP downregulation, CCUG-repeat toxicity and CCUG-repeat-encoded peptides in DM2. Using a Drosophila model with muscle-specific CNBP knockdown, we demonstrated that locomotor defects caused by CNBP loss are fully rescued by re-expression of either Drosophila or human CNBP, underscoring the evolutionary conservation of CNBP function. Notably, we found that the levels of ODC and polyamines are reduced upon dCNBP depletion, and most importantly in DM2 patients. Mechanistically, we provide evidence that dCNBP controls polyamine metabolism through regulating ODC translation.
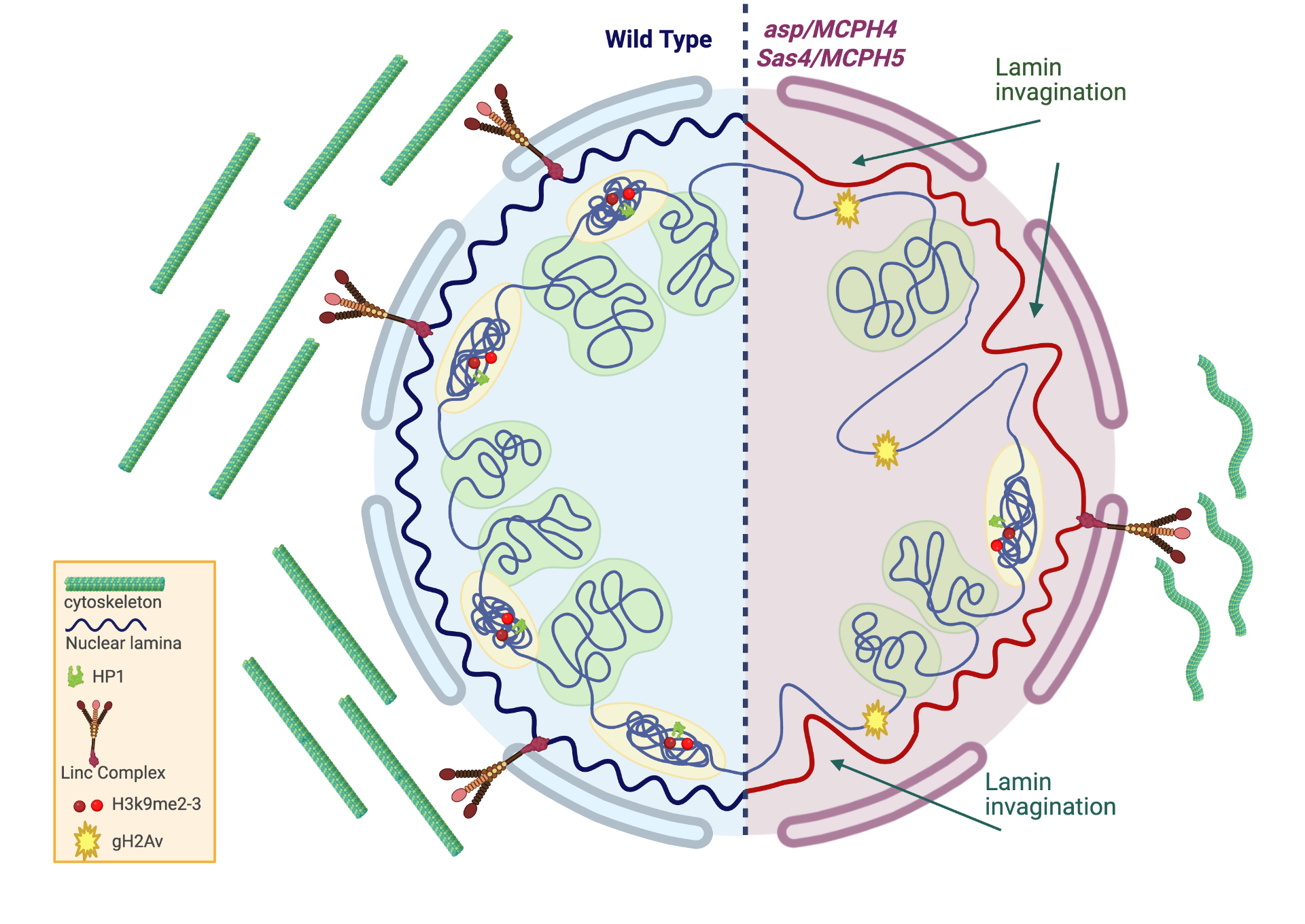
Drosophila as model system for genetic primary microcephaly
Primary microcephaly is an invalidating condition characterized by a reduced number of neurons, resulting from alterations of the delicate balance between proliferation, differentiation and death. In the developing brain, the proteins encoded by MCPH genes are required for maintaining genome stability, ensuring a precise temporal order of gene expression patterns and maintaining a correct balance in cell fate commitment. We are studying whether DNA damage, apoptosis, altered chromatin organization, abnormal transcription profiles and altered differentiation are consequences of the reduced function of the known MCPH genes, as compared to other genes that have not yet been involved as causal factors.

Drosophila for understanding the aging dependent locomotor decline
Aging progressively modifies the physiological balance of the organism, increasing susceptibility to degenerative diseases. However, the interrelationship between the aging process and disease-causing genes is unknown. The ribonuclear protein TDP-43 has been implicated in the pathophysiology of ALS, with genetic mutations being linked to the neurological symptoms of the disease. We are studying the contribution of TDP-43 gene regulation during aging in locomotor abilities. Our preliminary data indicate that the decrease in fly locomotor activity during aging is due to the downregulation of TDP-43, caused by an increase in methylation of histone H3K9me3 on the promoter region. These results pinpoint a possible novel role for TDP-43 in the regulation of locomotory senescence and suggest that other modulators of this genetic trait may play a similarly role in the pathogenesis of ALS.
